The first Coronavirus vaccines were approved by the FDA in late November 2020 and early December. They are reported to have 90 - 96% effectiveness. So far, only 3 individuals in the U.S. and U.K. have had an adverse (allergic). reaction to them (which was successfully treated.
There are at least 4 vaccines (many more in development);
Moderna and Pfizer COVID-19 vaccines have been shown to be 94 to 95 percent effective.
Side effects are expected to be similar to getting an annual flu shot or other vaccination. As noted above, with ANY vaccine, a very small number of people are allergic to some component of the vaccine.

The first batches are going (in December 2020) to frontline healthcare workers and residents of long-term care facilities. Current expectations are that it will be next June before most other people will be able to get a vaccination. At present, children under 16 are not included nor expected to need the vaccine
After all, the studies consistently show the risk to them is extremely low, and they are almost always asymptotic, if they even have an infection.
Pfizer's vaccine was approved for ages 16 and older. Moderna's vaccine has been approved for those 18 and older. The vaccines are not recommended for individuals who have experienced a serious reaction, such as anaphylaxis, to a prior dose of a COVID-19 vaccine or to any of its components. For information on vaccine components, refer to the manufacturers' package inserts from Pfizer and Moderna.
Here's what the CDC says:
There is still no point in it, unless the child has underlying medical conditions that put him/her at risk. Adolescents ages 16 and 17 years old may be vaccinated if they wish. In this age group, Pfizer trials included 153 participants (out of nearly 44,000 total participants). No safety concerns appeared. While data on safety and effectiveness for this age group is currently limited, the CDC says there' s no reason to believe the vaccine would work differently than it does for people who are 18 years and older.
The FDA does not allow it to be administered to anyone younger. Vaccine makers are still in the early stages of testing the vaccines on children.
Absolutely, yes, we should.
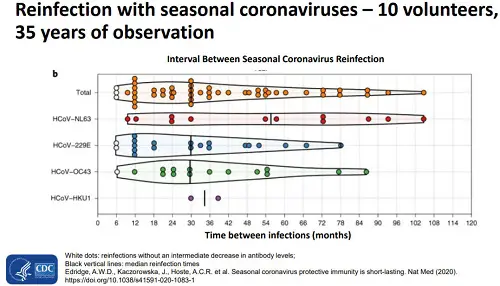 The chart at right shows CDC
research on coronavirus and time between reinfection after an infection. You can see the the shortest period of immunity is 6 months, but it
can be as long as 9 years, with the median being somewhere 2.5 to 3.5 year range.
The chart at right shows CDC
research on coronavirus and time between reinfection after an infection. You can see the the shortest period of immunity is 6 months, but it
can be as long as 9 years, with the median being somewhere 2.5 to 3.5 year range.
As of December 2020, no one really knows how long natural immunity may last after you get and recover from COVID-19. The CDC has not yet published any recommendations on when someone who' s previously had the virus should get vaccinated. They begrudgingly admit that reinfection is uncommon in the 90 days after initial infection, so testing and vaccination is not needed for at least 30 days after. If past vaccinations with other viruses is any indication, immunity could last 1 to 5 years.
So, it might be a good idea to get the vaccine when it is available to the general public around June - September 2021, even if you've had the virus. We do know immunity tends to fade over time.
But do not expect power-hungry governors or mayors to give up their dictatorship powers easily nor quickly. You may have to organize local groups to protest and demand the restoration of your Constitutional rights. As they say, "follow the science!" and the science says, that whole point of vaccines is to return to normal. Look at a hundred years of experience with vaccines as evidence: Polio, Tetanus, Chicken Pox, Measles, Mumps, Whooping Cough, etc.
Ways to save money AND help the environment:
Eat healthier AND save money: Instant Pot Duo Crisp 11-in-1 Air Fryer and Electric Pressure Cooker Combo with Multicooker Lids that Fries, Steams, Slow Cooks, Sautés, Dehydrates
Save water AND money with this showerhead adapter, it lets the water flow until the water is hot, then shuts off water flow until you restart it, ShowerStart TSV Hot Water Standby Adapter
Protect your health with these:
Mattress Dust mite-Bedbug protector, 100% Waterproof, Hypoallergenic, Zippered
Handheld Allergen Vacuum Cleaner with UV Sanitizing and Heating for Allergies and Pet, Kills Mite, Virus, Molds, True HEPA with Powerful Suction removes Hair, Dander, Pollen, Dust,
Immune Support Supplement with Quercetin, Vitamin C, Zinc, Vitamin D3
GermGuardian Air Purifier with UV-C Light and HEPA 13 Filter, Removes 99.97% of Pollutants
5 Stage Air Purifier, Features Ultraviolet Light (UVC), H13 True Hepa, Carbon, PCO, Smart Wifi, Auto Mode, Quiet, Removes 99.97% of Particles, Smoke, Mold, Pet Dander, Dust, Odors
Interesting Reads:
THE PREPPER'S CANNING & PRESERVING BIBLE: [13 in 1] Your Path to Food Self-Sufficiency. Canning, Dehydrating, Fermenting, Pickling & More, Plus The Food Preservation Calendar for a Sustainable Pantry
The Backyard Homestead: Produce all the food you need on just a quarter acre! Paperback
The Citizens' Guide to Geologic Hazards: A Guide to Understanding Geologic Hazards Including Asbestos, Radon, Swelling Soils, Earthquakes, Volcanoes
The Uninhabitable Earth: Life After Warming
Book: The Sixth Extinction: An Unnatural History Paperback